For product information and pricing, Chat with sales agent:
or email us : sales@clirik.com
Click links below to see related products.

Calcium carbonate (CaCO₃) powder modification involves various techniques aimed at improving its properties for use in different industrial applications. These modifications can enhance characteristics such as dispersibility, hydrophobicity, particle size, compatibility with various matrices, and specific functional properties. Here are some common methods of modifying calcium carbonate powder:

Surface coating involves applying a layer of another material onto the surface of calcium carbonate particles. Common coating agents include:

Calcium Carbonate Powder Surface Coating Machine
l Stearic Acid: Enhances hydrophobicity and dispersibility in non-polar matrices such as plastics and rubber.
l Silane Coupling Agents: Improve compatibility with organic polymers, enhancing adhesion and mechanical properties.
l Titanate Coupling Agents: Increase compatibility with different matrices and improve processing characteristics.
l Polymeric Coatings: Can impart specific functionalities, such as improved dispersibility or chemical resistance.
Grinding and milling techniques are used to reduce the particle size of calcium carbonate.
Commonly used grinding equipment include Raymond mill, ultrafine mill...They can reduce the fineness of calcium carbonate powder to 50-3000 mesh.

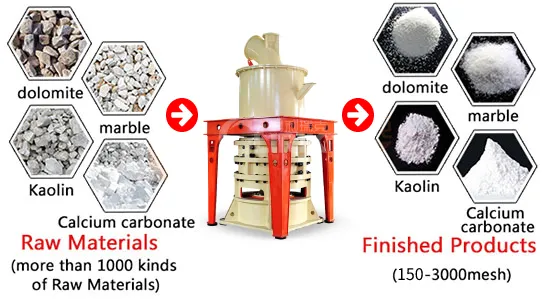
Fine particles can improve the performance of the powder in applications such as:
l Paints and Coatings: Providing better opacity, whiteness, and smooth texture.
l Plastics and Polymers: Enhancing mechanical properties and surface finish.
l Paper: Increasing brightness and smoothness.
Surface activation techniques increase the reactivity and surface area of calcium carbonate particles. These methods include:
l Acid Treatment: Enhances the surface area and can introduce active sites for further functionalization.
l Alkali Treatment: Modifies surface properties to improve compatibility with certain materials.
Functionalization introduces specific functional groups onto the surface of calcium carbonate particles, tailoring them for particular applications. Techniques include:
Silane Treatment: Adding organosilane groups to improve adhesion to organic materials.
Titanate Treatment: Introducing organotitanate groups to enhance compatibility and performance in composites.
Thermal treatment, such as calcination, can alter the crystal structure of calcium carbonate, potentially enhancing its properties for specific uses. This process can be used to produce different forms of calcium carbonate, such as:
Precipitated Calcium Carbonate (PCC): Offering higher purity and controlled particle size.
Ground Calcium Carbonate (GCC): Providing a more natural form with varying particle sizes.
Aspect | Ground Calcium Carbonate (GCC) | Precipitated Calcium Carbonate (PCC) |
Production Method | Mined and mechanically ground from natural sources such as limestone, chalk, or marble | Chemically synthesized by reacting calcium hydroxide with carbon dioxide |
Particle Size | Typically larger and less uniform; particle sizes vary depending on grinding process | Precisely controlled, finer, and more uniform; tailored to specific applications |
Particle Shape | Irregular and angular shapes due to mechanical grinding | Can be controlled to specific shapes (e.g., rhombohedral, scalenohedral) during precipitation |
Purity | Generally lower, containing natural impurities | Higher purity due to controlled synthesis and removal of impurities |
Brightness | Can vary; generally lower compared to PCC | Higher brightness and whiteness due to high purity and controlled synthesis |
Production Cost | Lower due to simpler mechanical processing | Higher due to complex chemical synthesis process |
Applications | Construction materials, low-cost fillers in plastics, paper, paints, rubber, and adhesives | High-quality fillers and coatings in paper, pharmaceuticals, food, high-performance plastics, and specialized paints and coatings |
Quality Control | Less control over particle size and purity | High control over particle size, shape, and purity |
Specific Advantages | Cost-effective for bulk applications, natural composition | Tailored properties for high-performance applications, higher performance due to controlled characteristics |
Co-precipitation involves precipitating calcium carbonate in the presence of other substances, creating modified particles with enhanced properties. This method can be used to introduce dopants or create composite materials with tailored characteristics.
Modified calcium carbonate powder is used across various industries due to its enhanced properties:
l Plastics and Polymers: As a filler to improve mechanical properties, reduce costs, and enhance processing.
l Paints and Coatings: For improved opacity, whiteness, and weather resistance.
l Paper: As a filler and coating pigment to enhance brightness, smoothness, and printability.
l Adhesives and Sealants: To improve rheology, mechanical properties, and reduce shrinkage.
l Pharmaceuticals: As an excipient with controlled release properties and improved bioavailability.
l Rubber: Enhancing tensile strength, abrasion resistance, and processability.
Modifying calcium carbonate powder is essential for optimizing its performance in a wide range of industrial applications. By selecting appropriate modification techniques, manufacturers can tailor calcium carbonate to meet specific requirements, enhancing the functionality and value of the final products.
